How do we tell elements apart from each other? Find out in this video from the Properties of Matter chapter of the Virtual School GCSE Chemistry.
00:00:05,920 -- 00:00:09,550
When you look at the periodic table you will
see that each element has its own box, and
00:00:09,550 -- 00:00:16,550
within that box, you will find two numbers.
The atomic number, or proton number, and the
00:00:17,480 -- 00:00:24,480
mass number, but what do these numbers mean?
The atomic number has the symbol 'z',
00:00:27,220 -- 00:00:33,280
this number tells you how many protons are
in one atom of an element. The number is always
00:00:33,280 -- 00:00:40,280
the same for all atoms of a particular element.
Atoms of different elements have different
00:00:40,580 -- 00:00:45,250
atomic numbers, meaning they have different
numbers of protons.
00:00:45,250 -- 00:00:52,250
For example, an atom of Hydrogen has an atomic
number of 1 because it has 1 proton, but an
00:00:53,720 -- 00:01:00,720
atom of Oxygen has and atomic number of 8
because it has 8 protons.
00:01:01,659 -- 00:01:07,640
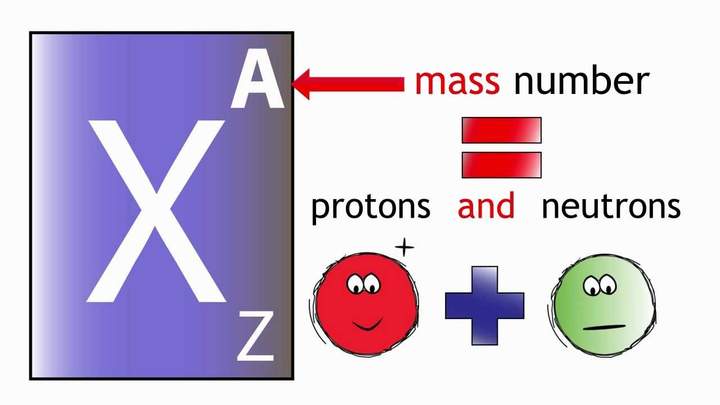
The next number we look at is the mass number.
The mass number has the symbol A.
00:01:07,640 -- 00:01:14,640
The mass number tells you how many protons
and neutrons are in one atom of an element.
00:01:16,439 -- 00:01:23,439
We need to remember that Protons and Neutrons
each have a relative mass of 1 and that Electrons
00:01:25,130 -- 00:01:31,030
are so small, that their mass does not need
to be considered in the mass number of an atom.
00:01:31,030 -- 00:01:38,030
So if we know the mass number of an element,
and we know the atomic number, we can calculate
00:01:38,149 -- 00:01:44,299
the number of Neutrons in an atom of a particular
element.
00:01:44,299 -- 00:01:51,299
So the Mass Number = The Atomic Number + the
Number of Neutrons. The Atomic Number is just the number of Protons and Atoms, therefore,
00:01:55,990 -- 00:02:02,990
The Mass Number = protons + neutrons.
So if we take oxygen,
00:02:04,090 -- 00:02:11,090
Oxygen has a mass number of 16 and 8 Protons, how many neutrons does it have?
00:02:12,530 -- 00:02:19,530
Remember, Mass Number = protons + neutrons
We can rearrange this to show that
00:02:20,569 -- 00:02:27,569
Neutrons = Mass Number - Atomic Number.
Neutrons = 16 - 8
00:02:31,189 -- 00:02:36,700
= 8
Oxygen therefore has 8 neutrons.
00:02:36,700 -- 00:02:39,409
Let take another example,
00:02:39,409 -- 00:02:46,409
Lithium has a mass number of 7 and an atomic
mass of 3, how many neutrons does it have?
00:02:47,040 -- 00:02:52,090
Mass Number = protons + neutrons
We can rearrange this to show that
00:02:52,090 -- 00:02:59,090
Neutrons = Mass Number - Atomic Number
Neutrons = 7- 3
00:02:59,299 -- 00:03:05,640
= 4
Lithium therefore has 4 neutrons.
00:03:05,640 -- 00:03:12,230
So the atomic number is the number of protons
in an atom and the mass number is the number
00:03:12,230 -- 00:03:14,719
of protons and neutrons in an atom.
This is part of our sciences pilot for the virtual school. We would appreciate any comments or feedback.
Want to learn more?
Visit: www.thevirtualschool.com
This video is distributed under a Creative Commons License:
Attribution-NonCommercial-NoDerivs
CC BY-NC-ND
Also, make sure to follow us on Twitter: https://twitter.com/virtualschooluk
and subscribe to interesting posts on Edutech and ICT4E on http://thevirtualschool.wordpress.com/
00:00:05,920 -- 00:00:09,550
When you look at the periodic table you will
see that each element has its own box, and
00:00:09,550 -- 00:00:16,550
within that box, you will find two numbers.
The atomic number, or proton number, and the
00:00:17,480 -- 00:00:24,480
mass number, but what do these numbers mean?
The atomic number has the symbol 'z',
00:00:27,220 -- 00:00:33,280
this number tells you how many protons are
in one atom of an element. The number is always
00:00:33,280 -- 00:00:40,280
the same for all atoms of a particular element.
Atoms of different elements have different
00:00:40,580 -- 00:00:45,250
atomic numbers, meaning they have different
numbers of protons.
00:00:45,250 -- 00:00:52,250
For example, an atom of Hydrogen has an atomic
number of 1 because it has 1 proton, but an
00:00:53,720 -- 00:01:00,720
atom of Oxygen has and atomic number of 8
because it has 8 protons.
00:01:01,659 -- 00:01:07,640
The next number we look at is the mass number.
The mass number has the symbol A.
00:01:07,640 -- 00:01:14,640
The mass number tells you how many protons
and neutrons are in one atom of an element.
00:01:16,439 -- 00:01:23,439
We need to remember that Protons and Neutrons
each have a relative mass of 1 and that Electrons
00:01:25,130 -- 00:01:31,030
are so small, that their mass does not need
to be considered in the mass number of an atom.
00:01:31,030 -- 00:01:38,030
So if we know the mass number of an element,
and we know the atomic number, we can calculate
00:01:38,149 -- 00:01:44,299
the number of Neutrons in an atom of a particular
element.
00:01:44,299 -- 00:01:51,299
So the Mass Number = The Atomic Number + the
Number of Neutrons. The Atomic Number is just the number of Protons and Atoms, therefore,
00:01:55,990 -- 00:02:02,990
The Mass Number = protons + neutrons.
So if we take oxygen,
00:02:04,090 -- 00:02:11,090
Oxygen has a mass number of 16 and 8 Protons, how many neutrons does it have?
00:02:12,530 -- 00:02:19,530
Remember, Mass Number = protons + neutrons
We can rearrange this to show that
00:02:20,569 -- 00:02:27,569
Neutrons = Mass Number - Atomic Number.
Neutrons = 16 - 8
00:02:31,189 -- 00:02:36,700
= 8
Oxygen therefore has 8 neutrons.
00:02:36,700 -- 00:02:39,409
Let take another example,
00:02:39,409 -- 00:02:46,409
Lithium has a mass number of 7 and an atomic
mass of 3, how many neutrons does it have?
00:02:47,040 -- 00:02:52,090
Mass Number = protons + neutrons
We can rearrange this to show that
00:02:52,090 -- 00:02:59,090
Neutrons = Mass Number - Atomic Number
Neutrons = 7- 3
00:02:59,299 -- 00:03:05,640
= 4
Lithium therefore has 4 neutrons.
00:03:05,640 -- 00:03:12,230
So the atomic number is the number of protons
in an atom and the mass number is the number
00:03:12,230 -- 00:03:14,719
of protons and neutrons in an atom.
This is part of our sciences pilot for the virtual school. We would appreciate any comments or feedback.
Want to learn more?
Visit: www.thevirtualschool.com
This video is distributed under a Creative Commons License:
Attribution-NonCommercial-NoDerivs
CC BY-NC-ND
Also, make sure to follow us on Twitter: https://twitter.com/virtualschooluk
and subscribe to interesting posts on Edutech and ICT4E on http://thevirtualschool.wordpress.com/
Video
Enlace externo
Descripción
Atomic number and mass number | Chemistry | Fuse School
Clasificaciones
Formato
Cursos / Niveles
Asignaturas / Ambitos
Eje
Licenciamiento
Licencia Youtube
Objetivos de aprendizaje del recurso
Objetivo de aprendizaje CN08 OA 14Basal CN08 OA 14
Usar la tabla periódica como un modelo para predecir las propiedades relativas de los elementos químicos basados en los patrones de sus átomos, considerando:
- El número atómico.
- La masa atómica.
- La conductividad eléctrica.
- La conductividad térmica.
- El brillo.
- Los enlaces que se pueden formar.